Top Tests
The State Hygienic Laboratory provides environmental and public health testing for all 99 Iowa counties. (Iowa analyses numbers by county are based on the addresses of those who submit testing requests, including health care providers.)
Under the federal Clinical Laboratory Improvement Amendments of 1988, any laboratory or facility that performs laboratory testing of human specimens for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the assessment of the health of human beings, is required to obtain a CLIA certificate and to meet certain requirements. This rule applies no matter whom the facility bills for the service, or whether it provides the service at no charge.
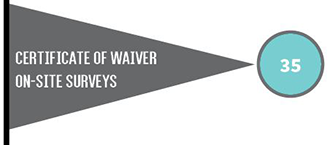
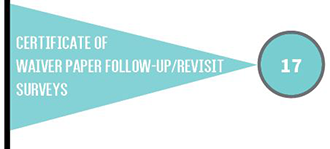
The type of CLIA certificate a laboratory must have depends on the type of testing the laboratory performs. Laboratories with certificates of compliance or accreditation are subject to routine inspections (surveys) every two years.
For more than 40 years, the Hygienic Laboratory, under contract with the Iowa Department of Inspections and Appeals, has provided the personnel to conduct laboratory surveys for programs required by the Clinical Laboratory Improvement Act (CLIA) of 1967 and the Clinical Laboratory Improvement Amendment of 1988. Since 2002, the laboratory has also been responsible for the administrative oversight of the CLIA laboratory program and served as the State Agency representative for the Center for Medicare and Medicaid Services (CMS) CLIA program.
The total number of health care facilities in Iowa with CLIA certificates increased from 3,080 to 3,112. The number of certificates of compliance and waiver increased, while certificates of accreditation and provider performed microscopy (PPMP) decreased.
- Organized and hosted the 2015 CLIA Midwest Consortium Conference at the Hygienic Laboratory’s Center for Advancement of Laboratory Science in Coralville.
- Published four issues of CLIA Corner.
- Presented “Data Entry Questions and Answers” at the CLIA Midwest Consortium Conference.
- Presented “Top Ten CLIA Deficiencies and Navigating CLIA Websites” to the Mercy Health Network Managers Meeting and the CLMA Annual Spring Meeting.
- Participated in the “Principles of Documentation” presentation at the CLIA Midwest Consortium Conference.