Test Detail
Newborn Screen
Back to Test Directory| Test Description |
|
||||
|---|---|---|---|---|---|
| Screening for certain genetic, endocrine, and metabolic disorders that can affect a child’s long-term health or survival. Early detection, diagnosis, and intervention can prevent death or disability and enable children to reach their full potential. | |||||
| Panel Components | |||||
| Biotinidase Deficiency, Congenital Adrenal Hyperplasia, Congenital Hypothyroidism, CPT1A Arctic Variant (c.1436C>T) (Alaska only), Cystic Fibrosis, Expanded Screening Disorders, Galactosemia, Hemoglobinopathies, Krabbe Disease (Iowa and North Dakota only), MPS1 Disease (Iowa and North Dakota only), MPSII Disease (Iowa and North Dakota only), Pompe Disease (Iowa, North Dakota, and South Dakota only), Severe Combined Immunodeficiency, Spinal Muscular Atrophy, and X-linked Adrenoleukodystrophy (Iowa, North Dakota, and South Dakota only). | |||||
| Reflex Test(s) | |||||
| Cystic Fibrosis by Luminex xMAP Technology, Cystic Fibrosis by Sanger Sequencing, Hemoglobinopathies by High Pressure Liquid Chromatography, Krabbe Disease 2nd Tier NBS by LC-MS/MS, Mucopolysaccharidosis (MPS) 2nd Tier NBS by LC-MS/MS, Pompe Disease 2nd Tier NBS by Flow Injection Analysis-Tandem Mass Spectrometry | |||||
| Performed | Avg. Turnaround Time | Method | |||
| Ankeny 24/7 |
1 - 3 business days | Enzymatic Assay, Immunoassay, IRT Immunoassay, Isoelectric Focusing, Real-Time PCR, Tandem Mass Spectrometry | |||
| Fee | CPT Code(s) | ||||
| $190.50 (AK), $162.00 (IA), $129.00 (ND), $106.00 (SD) | S3620 | ||||
| Specimen Requirements | |||||
| Specimen Type: | Blood spot specimen | ||||
| Collection Instructions: |
 Call the Newborn Screening laboratory at 515-725-1630 to obtain a copy of this document or to get assistance in collection of the newborn screen specimen. |
||||
| Shipping: | |||||
| Ship a fully dried specimen in an envelope at 15-30°C (do not use plastic). Send via courier (preferred). If courier information or setup is required, please contact shl-courier@uiowa.edu or call 319-335-1143. If courier delivery is not available mail specimens overnight to: Iowa Newborn Screening Program, State Hygienic Laboratory, 2220 S. Ankeny Blvd., Ankeny, IA 50023-9093. If courier and overnight are not available mail specimens to: Iowa Newborn Screening Program, State Hygienic Laboratory, P.O. Box 249, Ankeny, IA 50021-0249. | |||||
| Temperature and Stability: | Room temperature; submit specimen within 24 hours of collection. | ||||
| Rejection Criteria: |
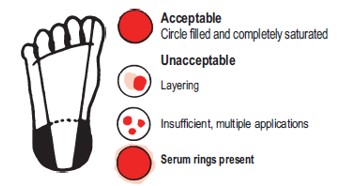
Specimens will be rejected if received under these conditions: Blood exhibiting layering, clotting, insufficient quantity, dilution, contamination, serum separation, applied to both sides of the filter paper, or didn't fully saturate through the filter paper. Sample collected on a damaged or expired filter paper collection device. Sample received at SHL more than 14 days after the sample was collected. |
||||
| Expected Results: | |||||
| Within Normal Limits, Presumptive Positive - Program staff will contact with recommendations, Presumptive Positive - Program staff will contact for second tier testing, Presumptive Positive - See second tier test result, Borderline - Resubmit newborn screen sample, Borderline - Program staff will contact with recommendations, Borderline - See second tier test result, Borderline - Program staff will contact for second tier testing, Poor Quality - Resubmit newborn screen sample, Early Collection - Resubmit newborn screen sample, Early Collection Unknown - Provide missing information, Transfused - Program staff will contact with recommendations, Transfused Unknown - Provide missing information, Inconclusive - Resubmit newborn screen sample, Unknown Weight - Elevated - Provide missing information, Possible Cystic Fibrosis - Program staff will contact with recommendations, Indeterminate results due to possible transfusion - Program staff will contact for clarification, numerous abnormal Hemoglobinopathies interpretations, Indeterminate - Program staff will contact with recommendations, Indeterminate - Resubmit newborn screen sample, Indeterminate with prior normal. No further action needed. Indeterminate due to Prematurity - Program staff will contact with recommendations, Abnormal Analyte - Program staff will contact with recommendations, Age at Collection Unknown - Provide missing information, Positive - see second tier results report attached to the sample in the OpenELIS Web Portal, Negative - see second tier results report attached to the sample in the OpenELIS Web Portal, See second tier results report attached to the sample in the OpenELIS Web Portal, Negative, Indeterminate - Arctic Variant, Homozygous-CPT1A Arctic Variant | |||||
| Comments | |||||
| This is a screening test and not indicated for stand-alone purposes; results should be used in conjunction with other available laboratory and clinical information. A false negative or a false positive result must always be considered when screening; therefore, clinical findings and status should be considered whenever interpreting laboratory results. Newborn reference values may not be applicable to older infants, thus screening results should be interpreted with caution in such cases. Information on the conditions screened is available at:
| |||||
